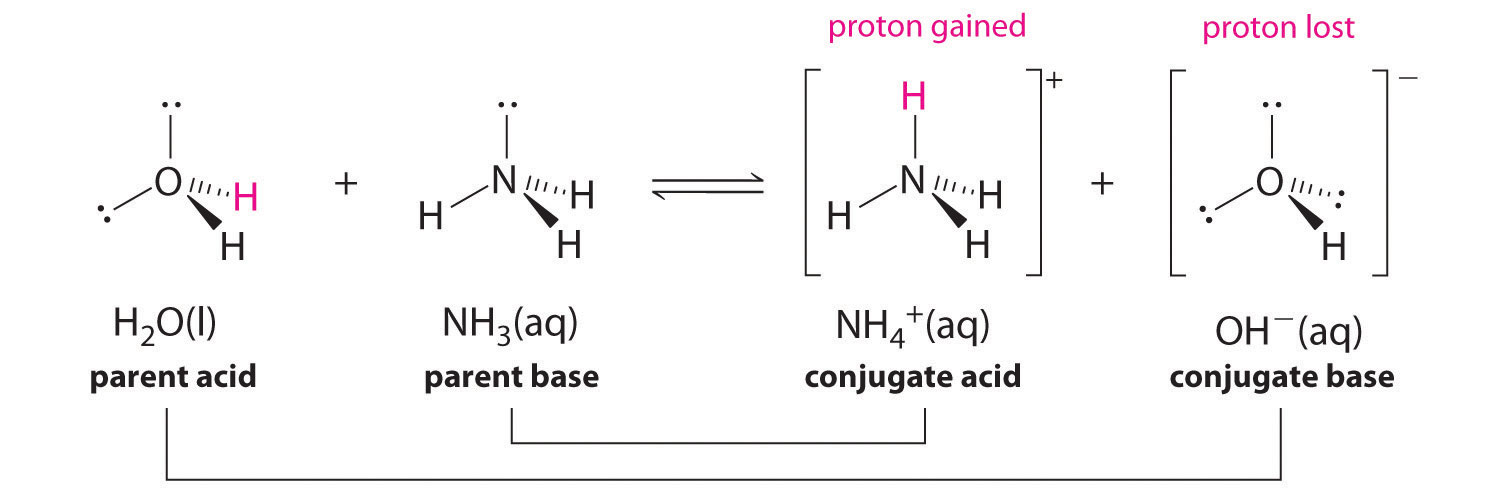
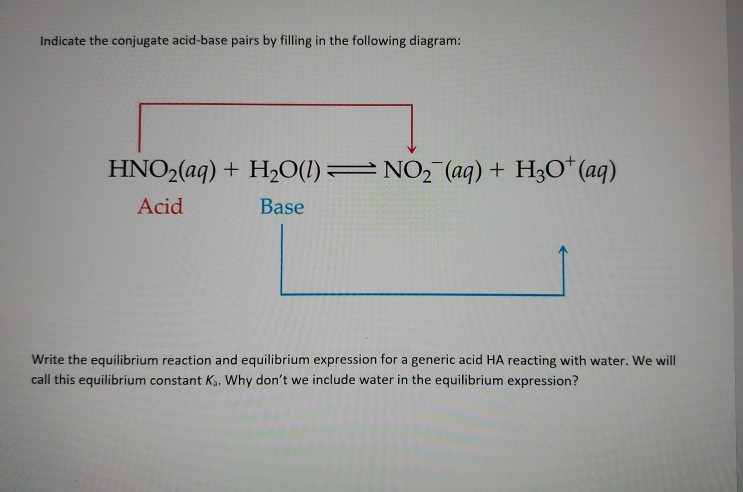
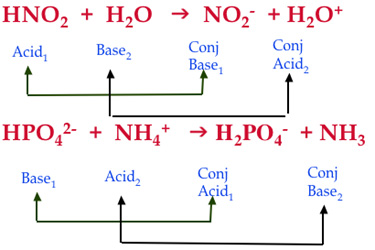
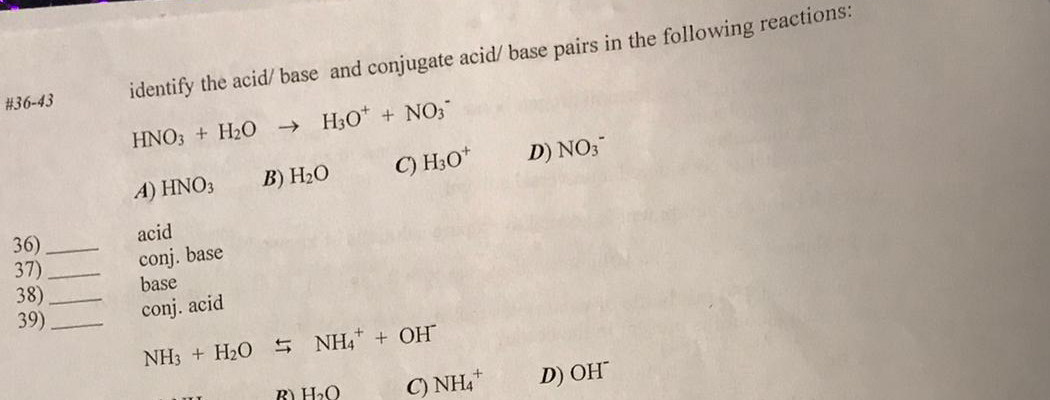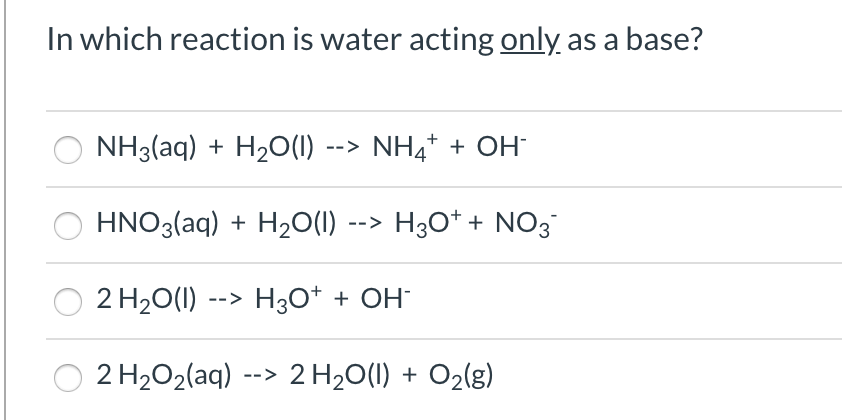For the reaction below, identify the Bronsted-Lowry acid, the Bronsted-Lowry base, the conjugate acid, and the conjugate base. HNO3(aq) + H2O(l) arrow H3O+(aq) + NO3-(aq) | Homework.Study.com

Question Video: Calculating the Concentration of Nitric Acid via Titrating against a Known Volume of Potassium Hydroxide | Nagwa

In which of the following pair of reactions first reaction is spontaneous while second reaction is non spontaneous?
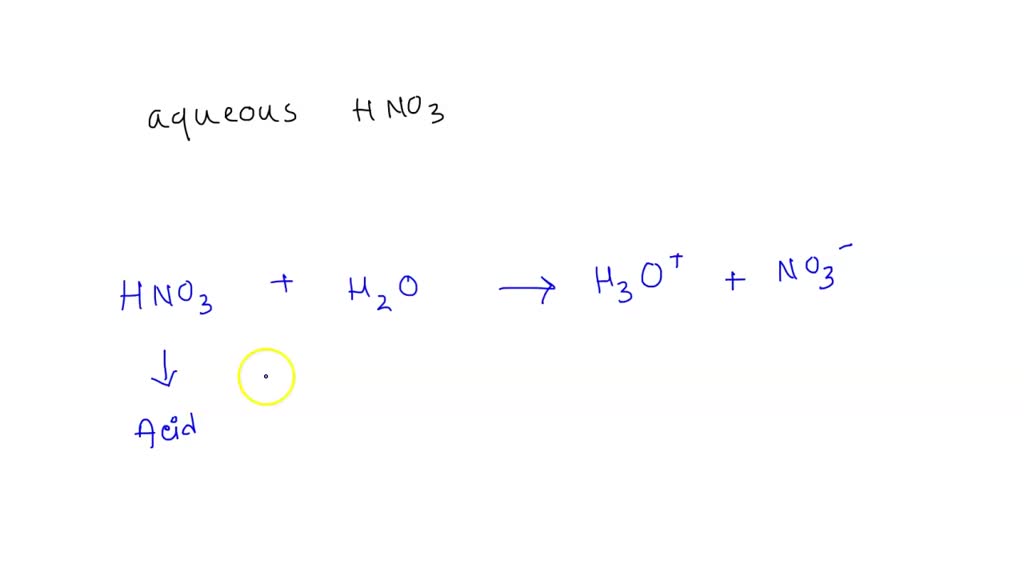
SOLVED: Which of the following would be the correct products for the following acid /base equation? HNO3 is the acid. HNO3 + H2O –> ? acid A.)H2NO3+ + H3O+ B.)H2NO3+ + OH-

SOLVED: Copy the following equation, and label the Bronsted-Lowry acid, its conjugate base, the Bronsted-Lowry base, and its conjugate acid. HNO3 + H2O → H30* + NO3

Nucleation of Mixed Nitric Acid–Water Ice Nanoparticles in Molecular Beams that Starts with a HNO3 Molecule | The Journal of Physical Chemistry Letters

Vibrational Signatures of HNO3 Acidity When Complexed with Microhydrated Alkali Metal Ions, M+·(HNO3)(H2O)n=5 (M = Li, K, Na, Rb, Cs), at 20 K | The Journal of Physical Chemistry A