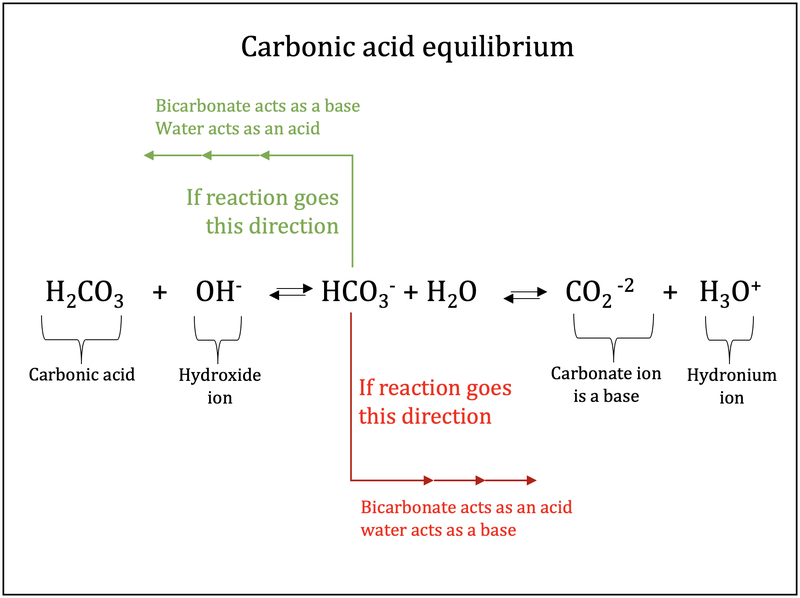
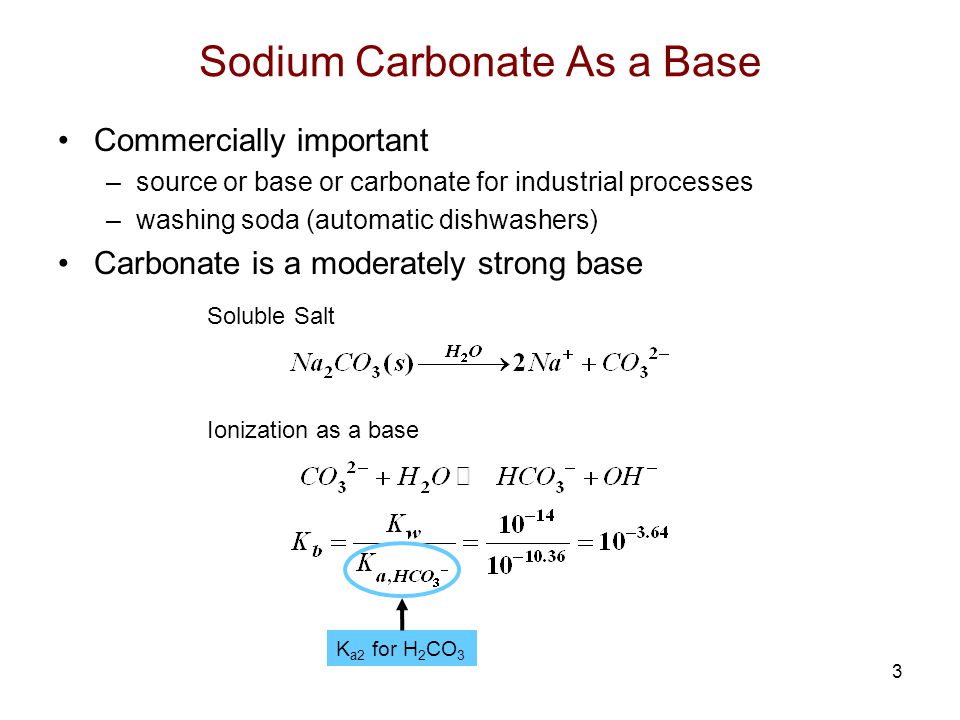
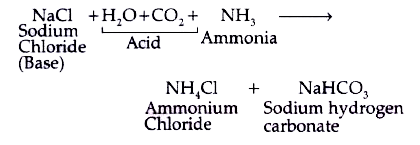Identify the acid and base which form sodium hydrogen carbonate write chemical equation in support of your answer state whether - Science - Acids Bases and Salts - 13500713 | Meritnation.com
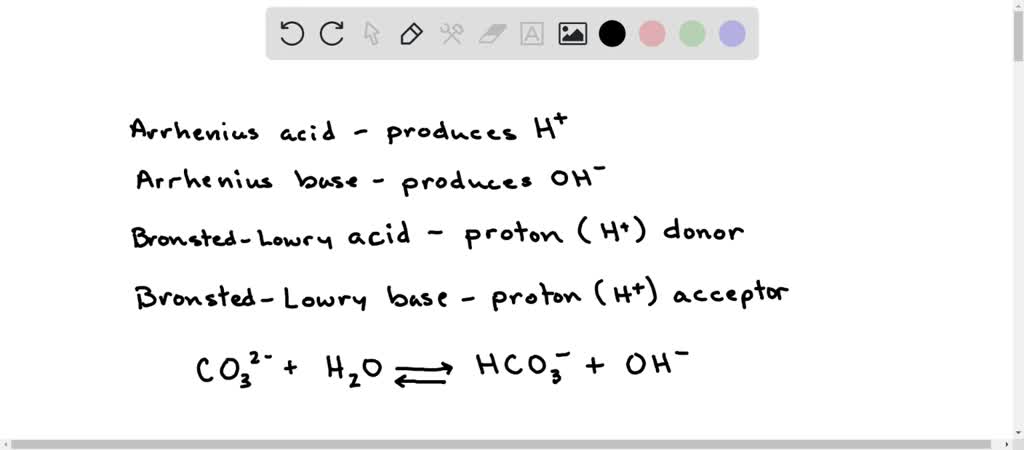
SOLVED: In the reaction CO3 + H2O â†' HCO3- + OH-, the carbonate ion is acting as a(n) Arrhenius base and a Bronsted-Lowry base.