![Potassium Phosphate Monobasic (KH2PO4, 500g) [CK02-500G] - $30.00 : Bioland Scientific, for Your Research Needs Potassium Phosphate Monobasic (KH2PO4, 500g) [CK02-500G] - $30.00 : Bioland Scientific, for Your Research Needs](https://www.bioland-sci.com/images/KH2PO4%20500G.jpg)
Potassium Phosphate Monobasic (KH2PO4, 500g) [CK02-500G] - $30.00 : Bioland Scientific, for Your Research Needs

Enhanced Second-Harmonic-Generation Response in a KH2PO4-Type Calcium Nitrate Carboxylate with Unusual Three-Dimensional Inorganic and Organic Connections | Inorganic Chemistry
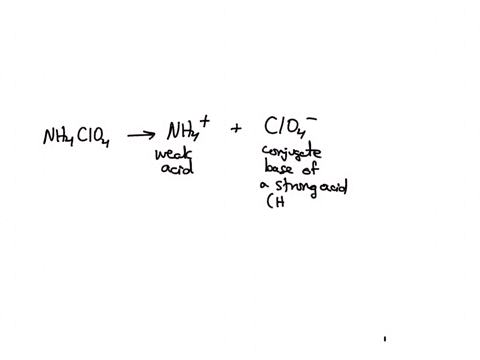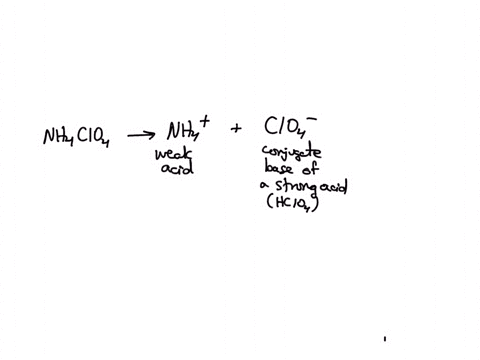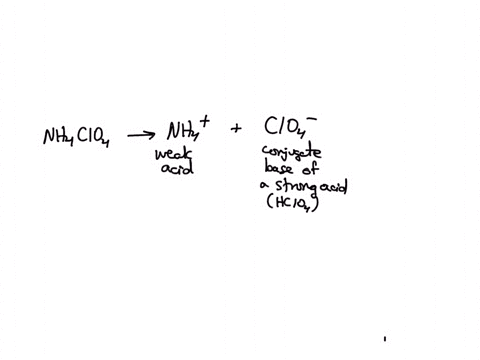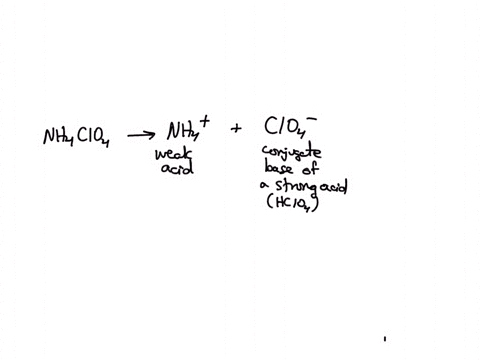
SOLVED: Polyprotic Acids (1) Is Dipotassium phosphate acidic, basic or neutral? (2) Is monopotassium phosphate (also called Potassium dihydrogen phosphate) acidic, basic or neutral? (3) Is Disodium citrate acidic, basic or neutral?

Calculate the pH of a buffer solution obtained by dissolving 25.0 g of KH2PO4(s) and 38.0 g of Na2HPO4(s) in water and then diluting to 1.00 L. | Homework.Study.com

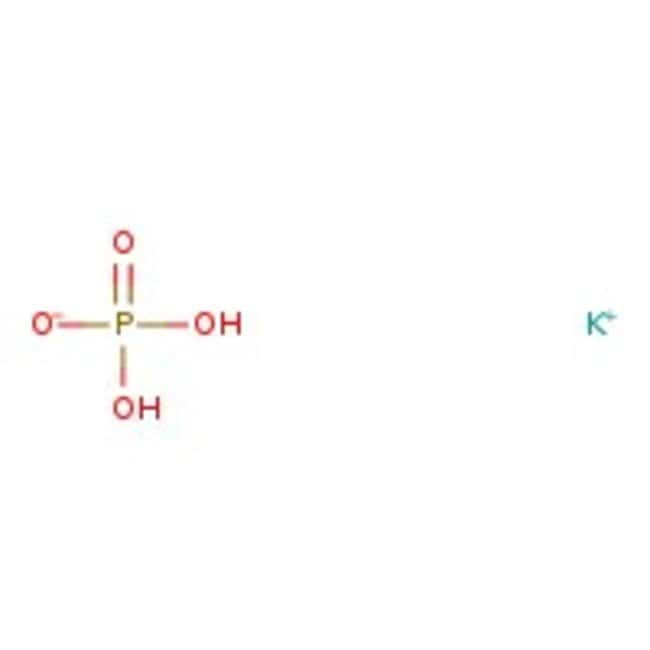




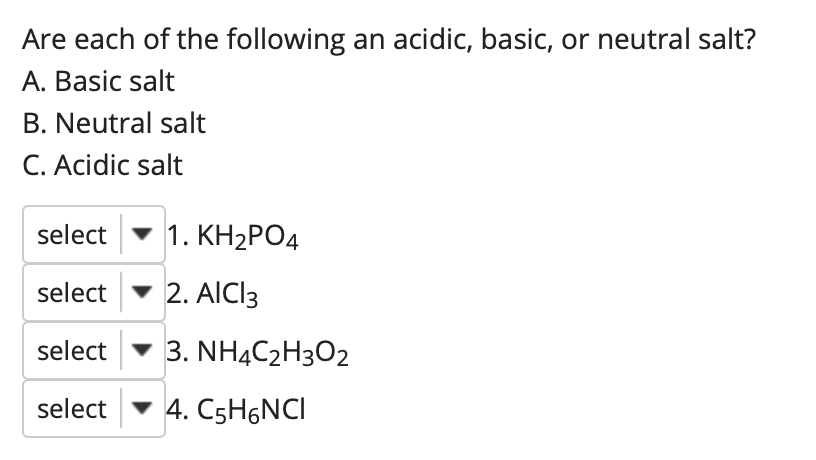
(358).jpg)