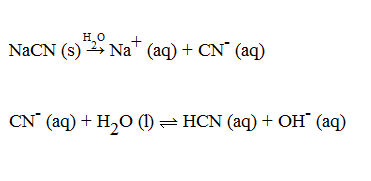Which pair of compounds will form a buffer in aqueous solution? NaCN and KCN HCl and NaOH NaCN - Home Work Help - Learn CBSE Forum

Predict if the solutions of the following salts are neutral, acidic or basic. NaCl, KBr, NaCN, NH4NO3,NaNO2 and KF

Draw the major product formed in the following reaction with NaCN and other reactants ethanol and water. | Homework.Study.com

SOLVED: 16- Consider a solution of 2.0 M HCN and 1.0 M NaCN (Ka for HCN = 6.2 10 10). Which of the following statements is true? A) The solution is not
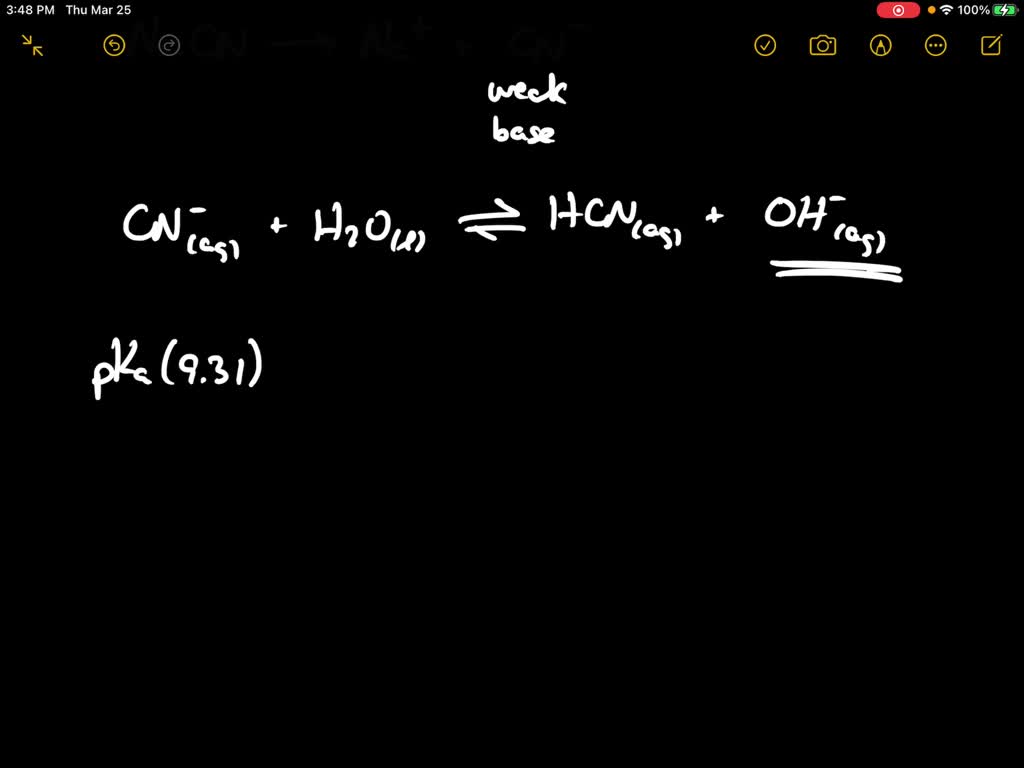
What is the % hydrolysis of NaCN in N/80 solution when dissociation constant for HCN is 1.3 x 10^-9 & Kw = 1 x 10^-14 - Sarthaks eConnect | Largest Online Education Community
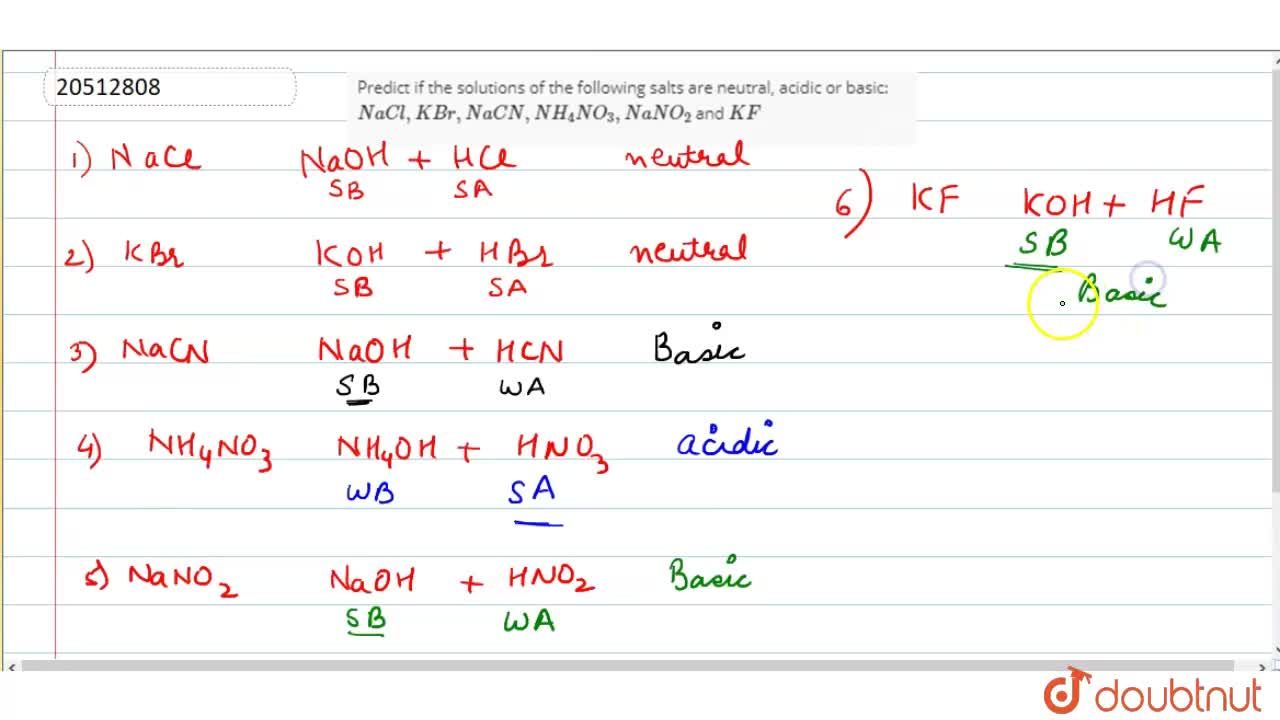
Predict if the solutions of the following salts are neutral, acidic or basic: NaCl, KBr, NaCN, NH(4)NO(3), NaNO(2) and KF
✓ Solved: An unknown salt is either NaCN, NaC2H3O2, NaF, NaCl, or NaOCl. When 0.100 mole of the salt...

Rational Catalysis Design on the Basis of Mechanistic Understanding: Highly Efficient Pd-Catalyzed Cyanation of Aryl Bromides with NaCN in Recyclable Solvents | Journal of the American Chemical Society