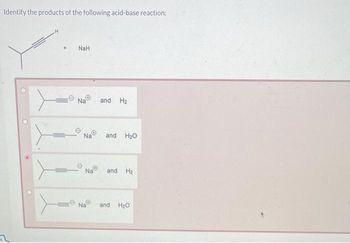
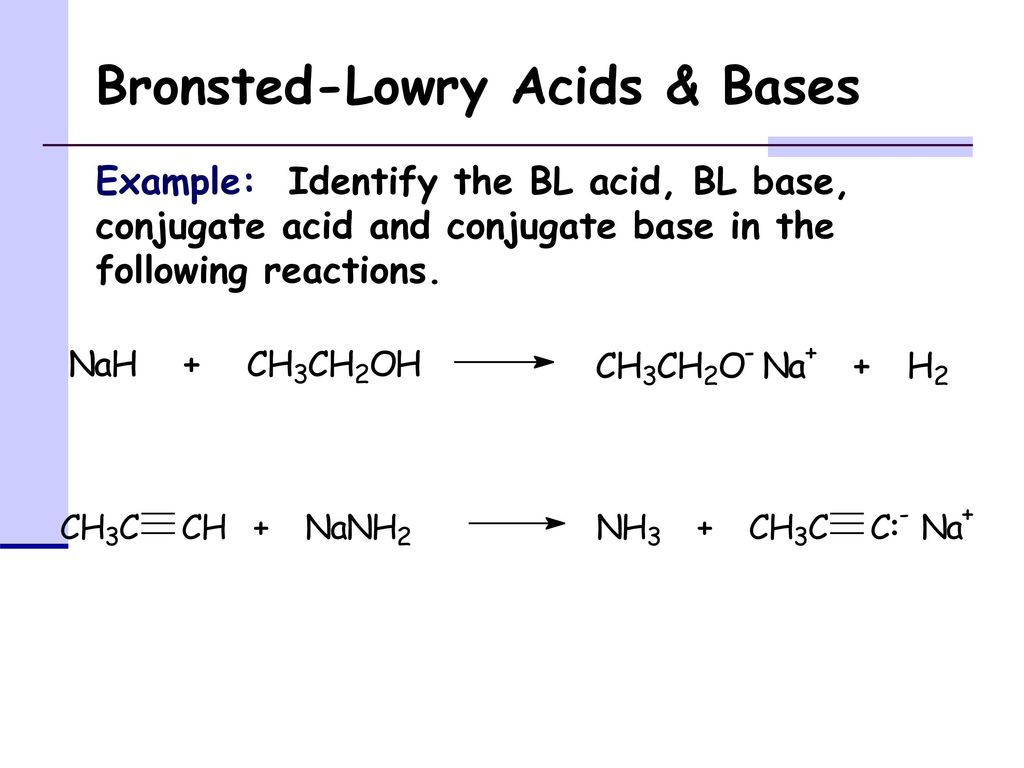
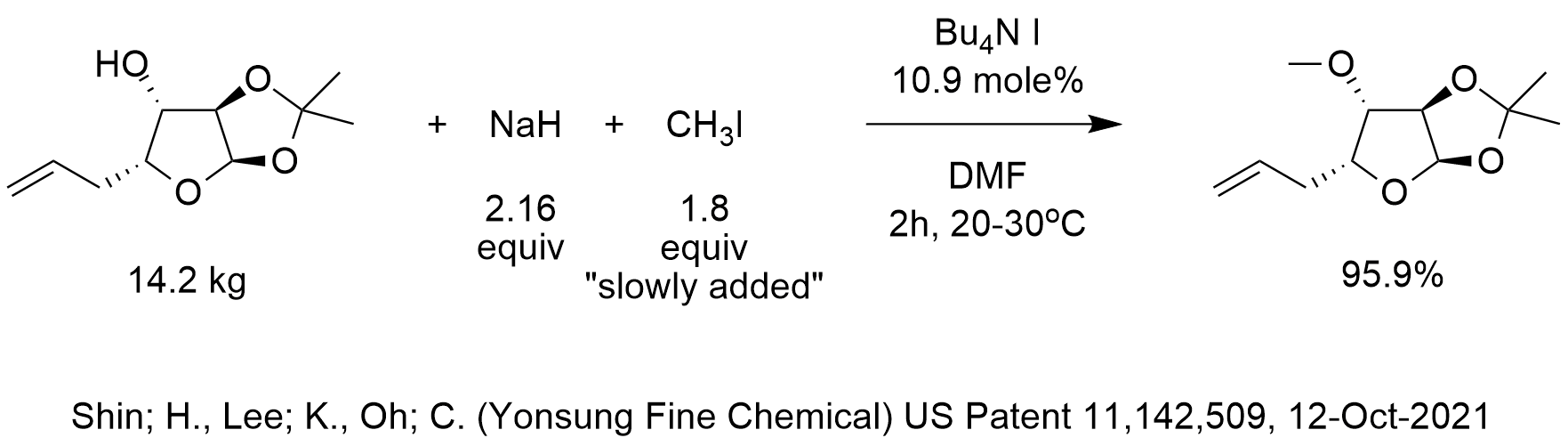
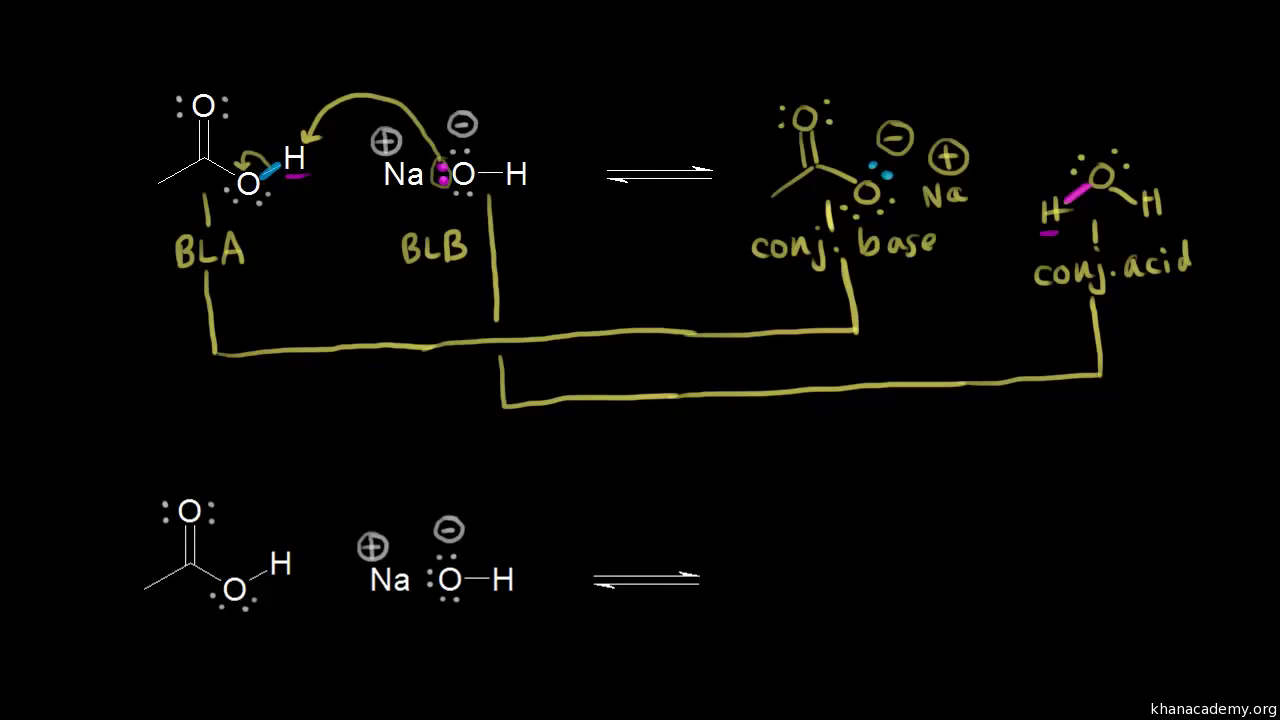
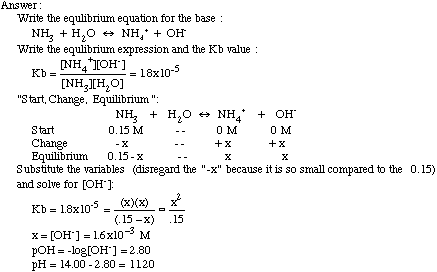The hydride ion ( H^ - ) is stronger base than OH^ - ion. Which of the following reaction will occurs if sodium hydride (NaH) is dissolved in water?

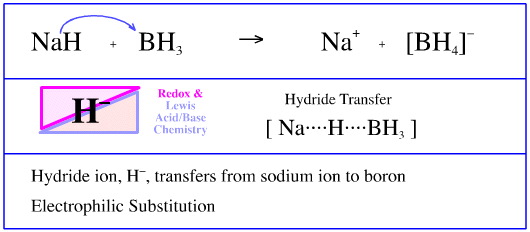
organic chemistry - Why can't carboxylic acid + NaH + tert-butyl bromide react to create an ester? - Chemistry Stack Exchange
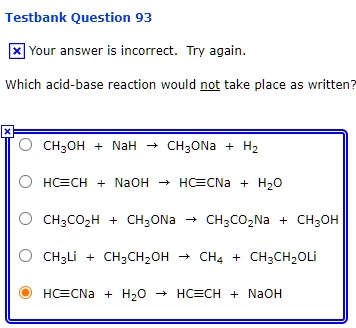
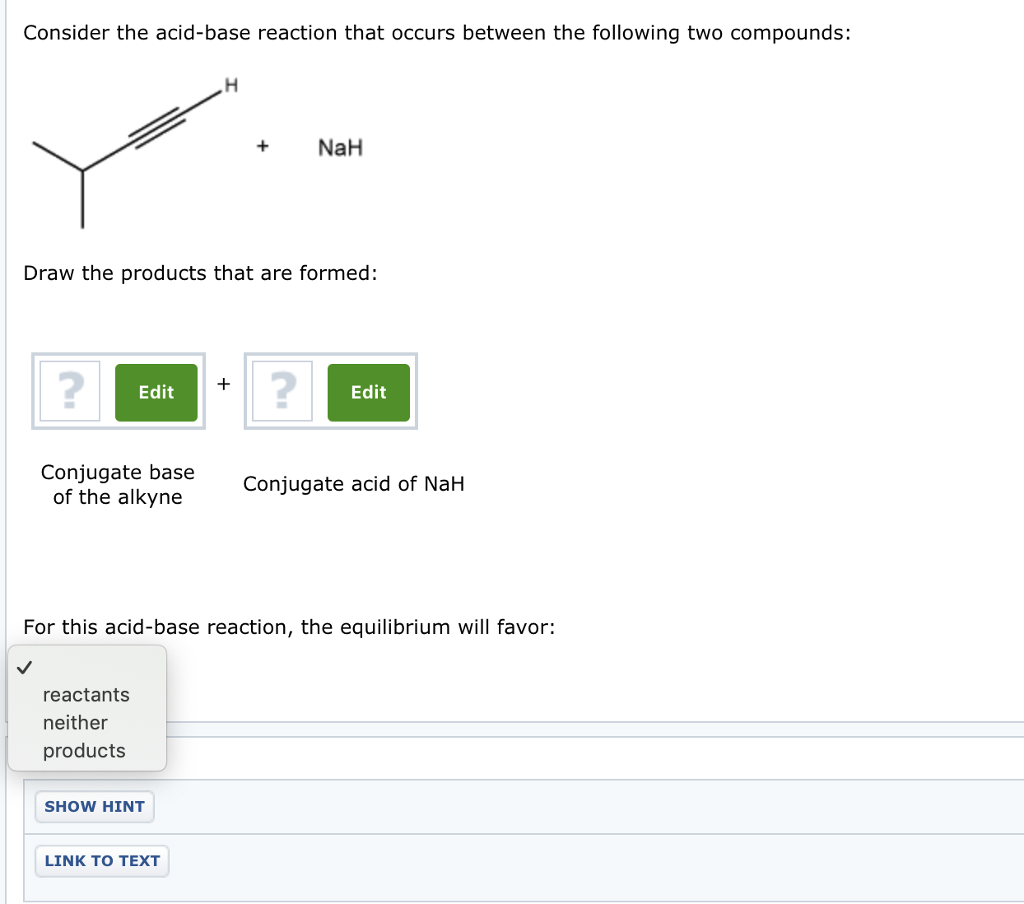
SOLVED: Testbank Question 93 Your answer incorrect Try again Which acid base reaction would not take place as written CHzOH Nah CHzONa HCCH NaOH HCCNa Hzo CH;COzH CHzONa CH;COzNa CHzOH CHzLi CHzCHzOH

organic chemistry - Can NaH open the epoxide ring to form alcohol? If so, how? - Chemistry Stack Exchange