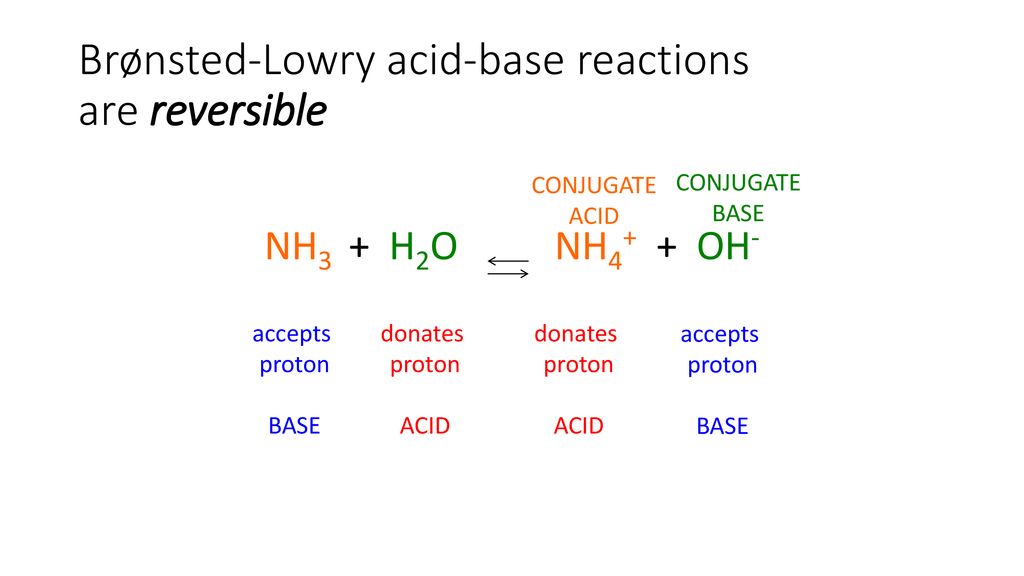
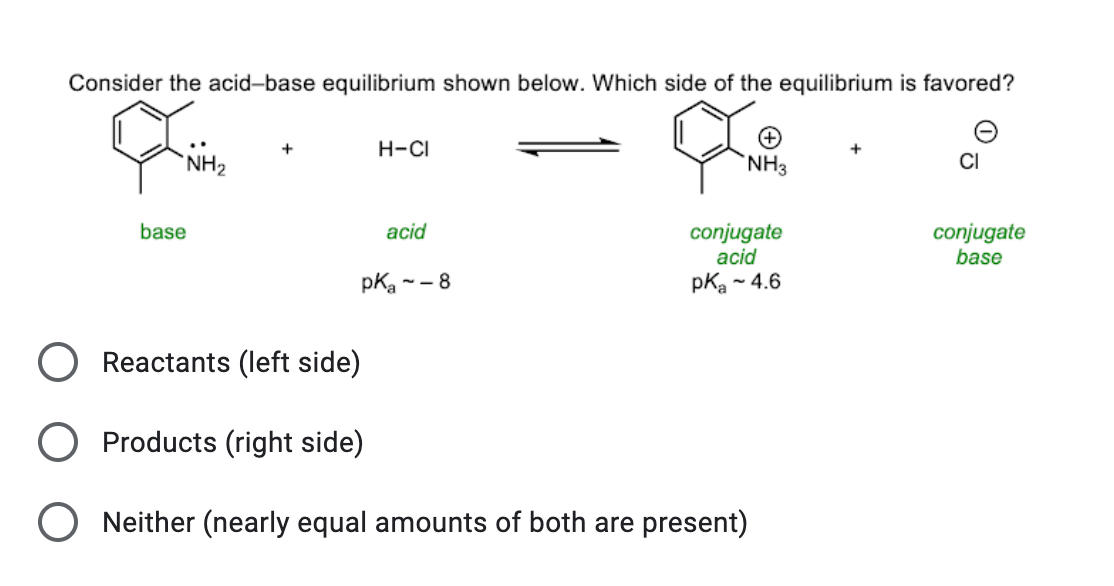
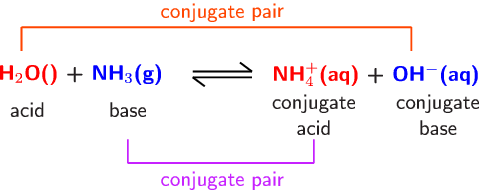
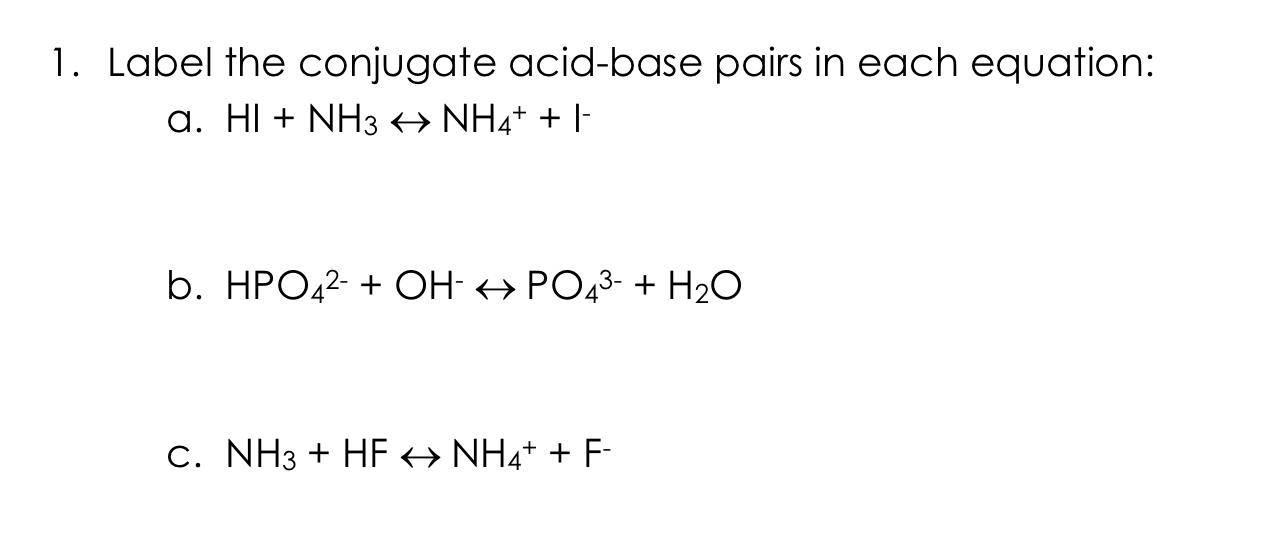NH3 is a weak base (Kb = 1.8 times 10^-5) and so the salt NH4Cl acts as a weak acid. What is the pH of a solution that is 0.050 M in
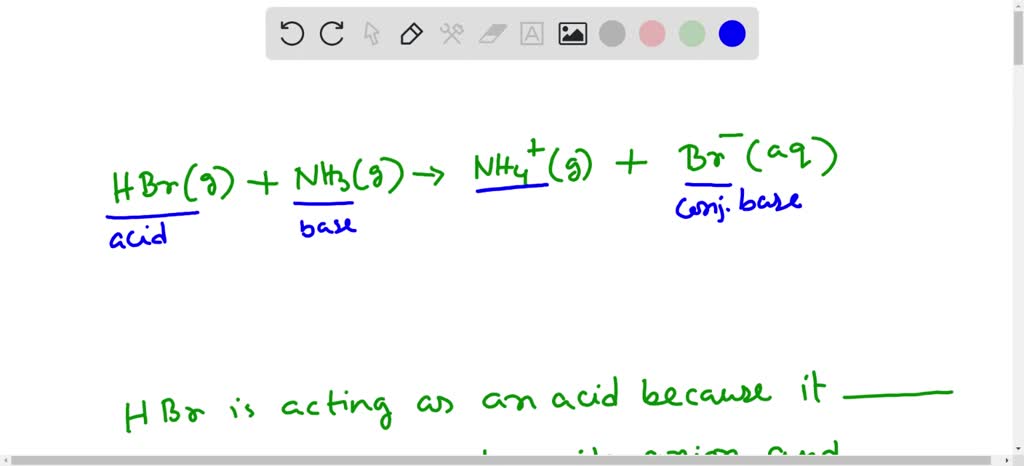
SOLVED: In the following acid-base reaction: HBr(g) + NH3(g) → NH4+(aq) + Br– (aq) HBr is acting as the acid, because it a proton to form bromide anion, and NH3 is acting
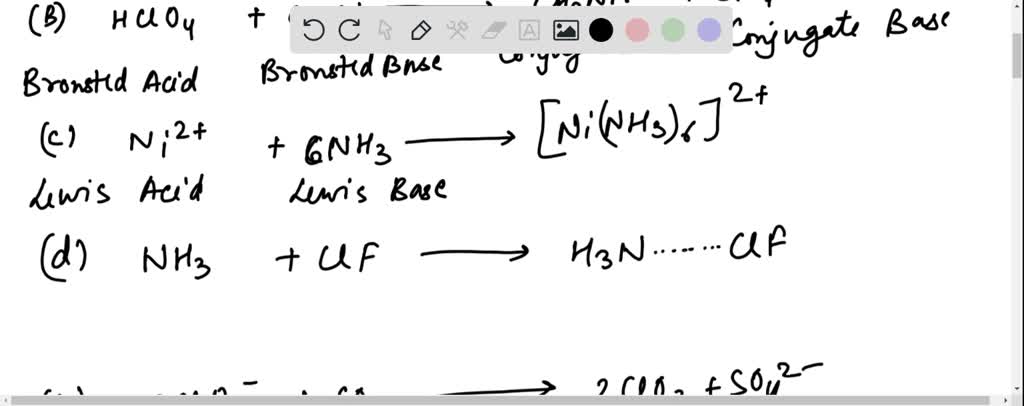
SOLVED: For each of the following reactions, identify the acid and the base by circling the acid and boxing the base. Also indicate whether Lux-Flood, Lewis or Bronsted-Lowry is the most appropriate

Identify the conjugate acid-base pairs in this equilibrium. NH3(aq) + H2S(aq) arrow HS-(aq) + NH4+(aq) | Homework.Study.com