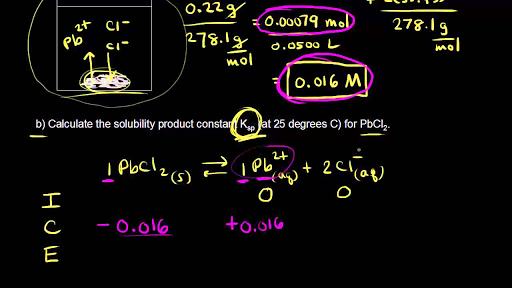
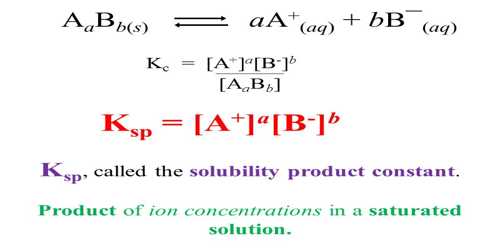
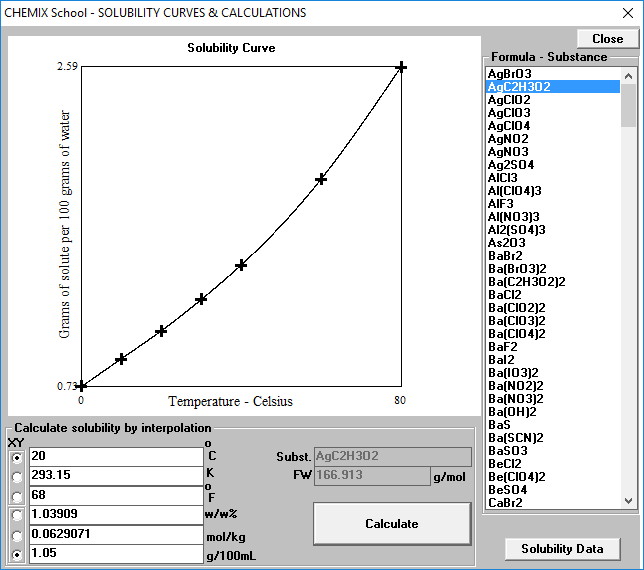
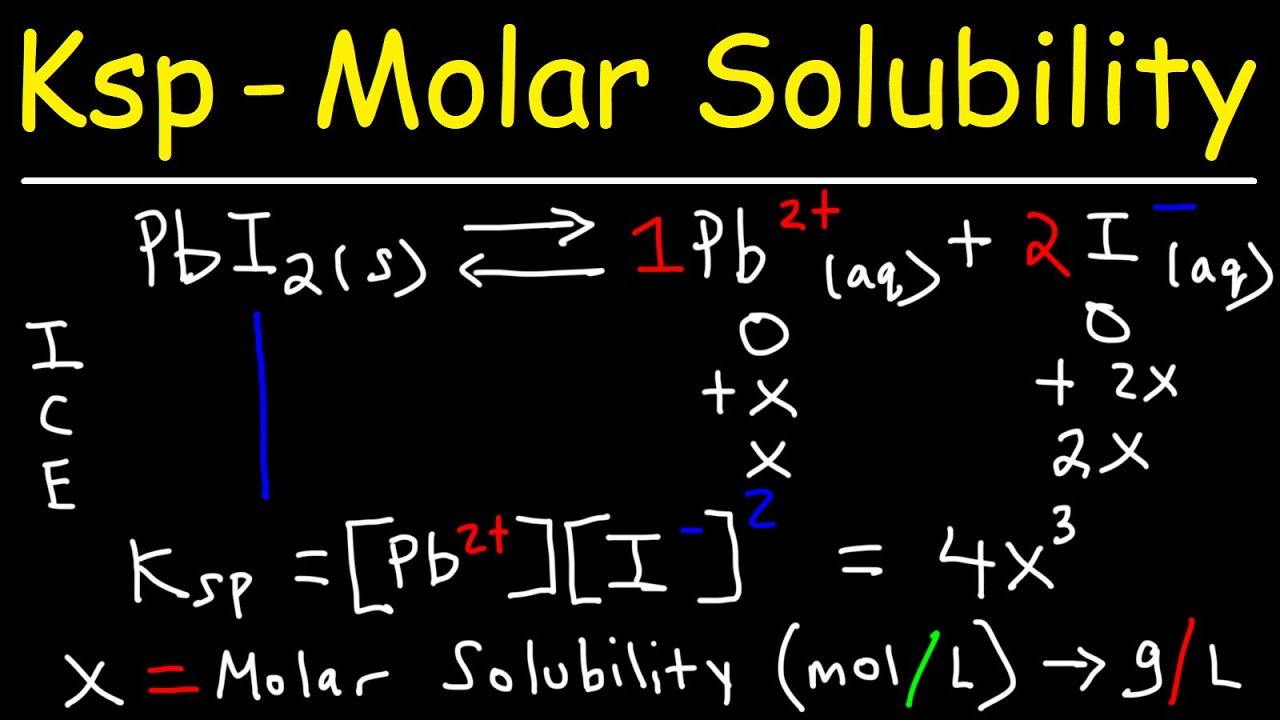
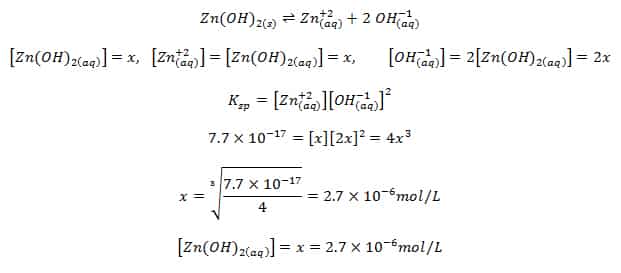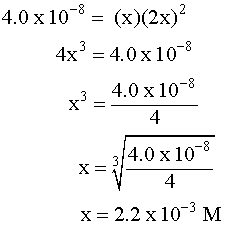✓ Solved: Calculate the molar solubility of SrC2O4 in a solution that has a fixed H3O^+ concentration...

SOLVED: Calculate the solubility (in g/L) of O2 in water at a partial pressure of O2 of 120 torr at 25C (K o2 = 780 atm*L/mol)
![SOLVED: #6 Calculate the total solubility of the basic drug € at pH 6.0. Additional information for drug C: SB (intrinsic solubility) = 0.8 mglmL pKa 8 2 St Sua (1+]OpH-pKa) Ans: 52 mglmL St = Sp (1+]OpKa-pH) SOLVED: #6 Calculate the total solubility of the basic drug € at pH 6.0. Additional information for drug C: SB (intrinsic solubility) = 0.8 mglmL pKa 8 2 St Sua (1+]OpH-pKa) Ans: 52 mglmL St = Sp (1+]OpKa-pH)](https://cdn.numerade.com/ask_images/7d7ca9e3ac22444fbaeef36ffd903405.jpg)
SOLVED: #6 Calculate the total solubility of the basic drug € at pH 6.0. Additional information for drug C: SB (intrinsic solubility) = 0.8 mglmL pKa 8 2 St Sua (1+]OpH-pKa) Ans: 52 mglmL St = Sp (1+]OpKa-pH)